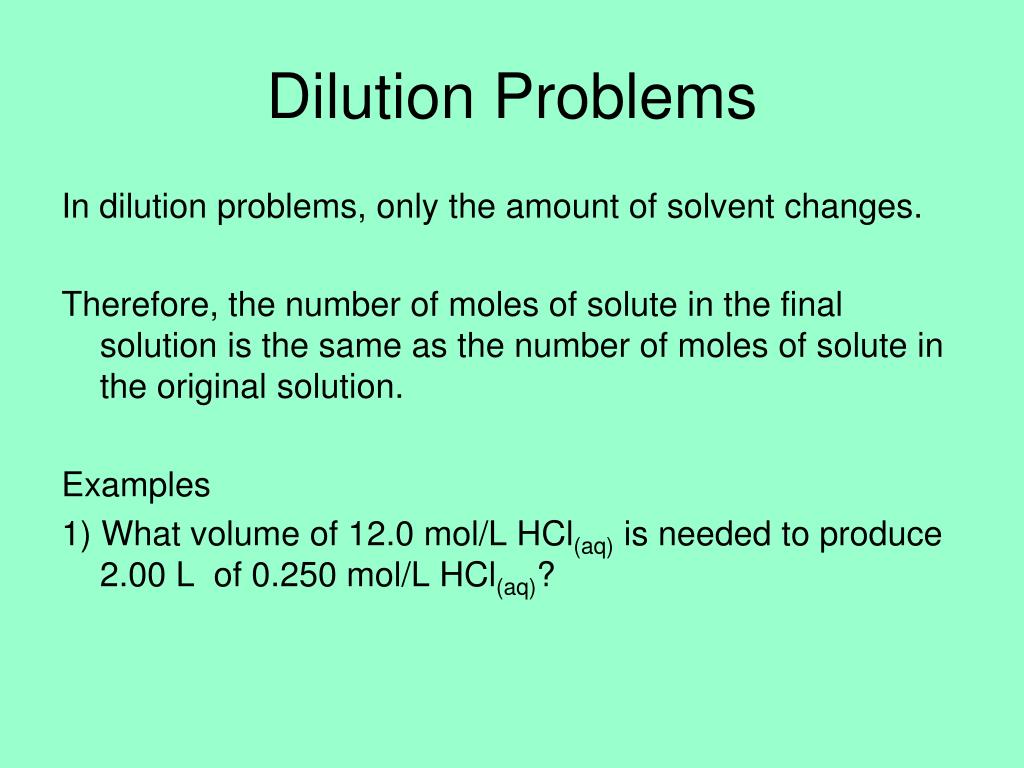
Dilution Problems Explained . a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. we can relate the concentrations and volumes before and after a dilution using the following equation: this chemistry video tutorial explains how to solve common dilution. Often, a worker will need to change the concentration of a. this process is known as dilution, because, relative to the solution from which it was prepared, the final. M₁v₁ = m₂v₂ where m₁. Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions.
from www.slideserve.com
this process is known as dilution, because, relative to the solution from which it was prepared, the final. learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. this chemistry video tutorial explains how to solve common dilution. we can relate the concentrations and volumes before and after a dilution using the following equation: M₁v₁ = m₂v₂ where m₁. Often, a worker will need to change the concentration of a.
PPT Solutions PowerPoint Presentation, free download ID4697528
Dilution Problems Explained Often, a worker will need to change the concentration of a. this chemistry video tutorial explains how to solve common dilution. Often, a worker will need to change the concentration of a. explain how concentrations can be changed in the lab. this process is known as dilution, because, relative to the solution from which it was prepared, the final. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. learn how to dilute and concentrate solutions. M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a dilution using the following equation: Understand how stock solutions are used in the laboratory.
From www.youtube.com
Molarity How to solve dilution problems Chemtutorfree YouTube Dilution Problems Explained this process is known as dilution, because, relative to the solution from which it was prepared, the final. M₁v₁ = m₂v₂ where m₁. learn how to dilute and concentrate solutions. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in. Dilution Problems Explained.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID4697528 Dilution Problems Explained M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a dilution using the following equation: Understand how stock solutions are used in the laboratory. this process is known as dilution, because, relative to the solution from which it was prepared, the final. Often, a worker will need to change the concentration of. Dilution Problems Explained.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and chemistry YouTube Dilution Problems Explained explain how concentrations can be changed in the lab. we can relate the concentrations and volumes before and after a dilution using the following equation: this chemistry video tutorial explains how to solve common dilution. Understand how stock solutions are used in the laboratory. this process is known as dilution, because, relative to the solution from. Dilution Problems Explained.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Problems Explained this process is known as dilution, because, relative to the solution from which it was prepared, the final. learn how to dilute and concentrate solutions. M₁v₁ = m₂v₂ where m₁. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. this chemistry video tutorial explains how to solve. Dilution Problems Explained.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Problems Explained this chemistry video tutorial explains how to solve common dilution. M₁v₁ = m₂v₂ where m₁. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a. a dilution is a process. Dilution Problems Explained.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems PowerPoint Presentation ID Dilution Problems Explained this chemistry video tutorial explains how to solve common dilution. M₁v₁ = m₂v₂ where m₁. Often, a worker will need to change the concentration of a. learn how to dilute and concentrate solutions. this process is known as dilution, because, relative to the solution from which it was prepared, the final. Understand how stock solutions are used. Dilution Problems Explained.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution Dilution Problems Explained M₁v₁ = m₂v₂ where m₁. this chemistry video tutorial explains how to solve common dilution. Understand how stock solutions are used in the laboratory. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Often, a worker will need to change the concentration of a. we can. Dilution Problems Explained.
From www.scribd.com
dilution tutorial and problems Significant Figures Concentration Dilution Problems Explained a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. M₁v₁ = m₂v₂ where m₁. this process is known as dilution, because, relative to the solution from which it was prepared, the final. explain how concentrations can be changed in the lab. Understand how stock solutions are. Dilution Problems Explained.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID2843233 Dilution Problems Explained Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a. M₁v₁ = m₂v₂ where m₁. this process is known as dilution, because, relative to the solution from which it was prepared, the final. this chemistry video tutorial explains how to. Dilution Problems Explained.
From www.studocu.com
Dilution problems I 12192022 Dilution problems I Please note for all dilution problems, use Dilution Problems Explained Understand how stock solutions are used in the laboratory. M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a dilution using the following equation: Often, a worker will need to change the concentration of a. this process is known as dilution, because, relative to the solution from which it was prepared, the. Dilution Problems Explained.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations YouTube Dilution Problems Explained we can relate the concentrations and volumes before and after a dilution using the following equation: Often, a worker will need to change the concentration of a. Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. M₁v₁ = m₂v₂ where m₁.. Dilution Problems Explained.
From www.youtube.com
GeeklyHub Dilution of Solution Dilution Calculations & Problems, Molarity YouTube Dilution Problems Explained learn how to dilute and concentrate solutions. this chemistry video tutorial explains how to solve common dilution. explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a. this process is known as dilution, because, relative to the solution from which it was prepared, the final. M₁v₁. Dilution Problems Explained.
From www.studypool.com
SOLUTION Dilution Problems Molarity Formula and Dilution Factor PAper Studypool Dilution Problems Explained a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. we can relate the concentrations and volumes before and after a dilution using the following equation: this process is known as dilution, because, relative to the solution from which it was prepared, the final. M₁v₁ = m₂v₂. Dilution Problems Explained.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Problems Explained M₁v₁ = m₂v₂ where m₁. Understand how stock solutions are used in the laboratory. this process is known as dilution, because, relative to the solution from which it was prepared, the final. this chemistry video tutorial explains how to solve common dilution. Often, a worker will need to change the concentration of a. learn how to dilute. Dilution Problems Explained.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems PowerPoint Presentation ID Dilution Problems Explained learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. this chemistry video tutorial explains how to solve common dilution.. Dilution Problems Explained.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution Dilution Problems Explained this chemistry video tutorial explains how to solve common dilution. this process is known as dilution, because, relative to the solution from which it was prepared, the final. we can relate the concentrations and volumes before and after a dilution using the following equation: Often, a worker will need to change the concentration of a. Understand how. Dilution Problems Explained.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID2843233 Dilution Problems Explained we can relate the concentrations and volumes before and after a dilution using the following equation: explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. M₁v₁ = m₂v₂ where m₁. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution. Dilution Problems Explained.
From www.slideserve.com
PPT Chemistry 100(02 ) Fall 2012 PowerPoint Presentation, free download ID2067461 Dilution Problems Explained learn how to dilute and concentrate solutions. we can relate the concentrations and volumes before and after a dilution using the following equation: a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. this chemistry video. Dilution Problems Explained.